U.S. FDA approves Amphastar Pharma's nasal spray for opioid overdose, Health News, ET HealthWorld
FDA
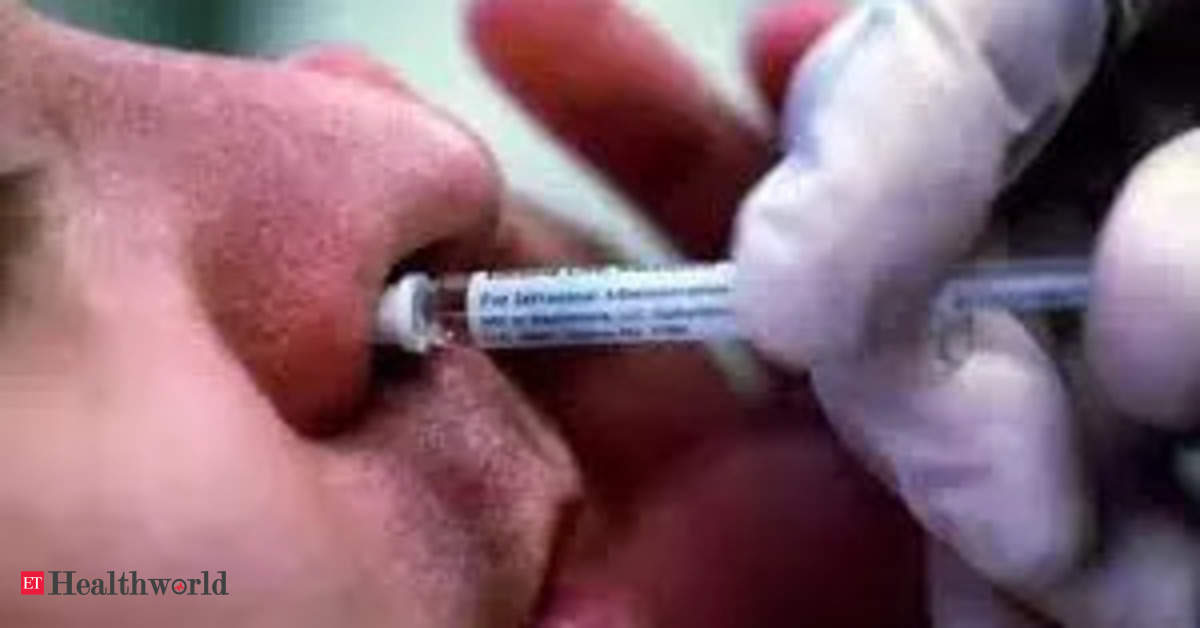
) had approved its nasal spray for emergency treatment of known or suspected opioid overdose.
The company's naloxone hydrochloride nasal spray can be delivered in one spray by intranasal administration - delivering 4 mg of the drug in adults and pediatric patients - for initial dosing, according to the FDA label. The regulator's approval allows its use only when prescribed.
The nod comes at a time when the agency is reviewing applications to also allow over-the-counter use of some naloxone-based drugs, including an application for Emergent Biosolutions' Narcan.
Late last year, the health regulator had said naloxone might be safe and effective for over-the-counter use in some forms, potentially paving the way for its use federally and encouraging more manufacturers to seek approval for prescription-free use.
More than 106,000 people died in the U.S. from drug-involved overdose in 2021, according to government data.
Follow and connect with us on Twitter, Facebook, Linkedin,
Thursday, March 9, 2023 at 7:20 am